Observe the color of the portion of blue litmus paper that was in contact with the solution. As you can see most weak acids can be identified by having hydrogen in front of the formula except for organic acids.

How To Determine If Acid Is Strong Or Weak Shortcut W Examples And Practice Problems Youtube
How to tell if the given acid is a weak acid.

. If we neutralize them with an strong base we will end up with a salt composed of a cation and an anion. Strength of acid and base. It becomes slightly acidic.
Distinguishing Between Strong and Weak Acids. They have high bond strength hence dont dissociate easily in an aqueous solution. The titration curve reveals the pKa of a weak acid.
Determine pH and concentrations of chemical species at any point in the titration of a weak acid by a strong base or a weak base by a strong acid. The NaOH is added as small increments of equal volume and at each step the pH of the solution is. The pH of the titration solution will be monitored using a pH meter.
It can be 1 ionised or 99 ionised but it is also categorised as a weak acid. A weak acid in other terms is an acid that is not strong. Identify the nature of a substance based on pH.
Acetic acid is a kind of acetic acid that CH 3 COOH. If 100 per cent does not dissociate it is a weak acid. Cut a one-inch piece of litmus paper.
And heres another way to look at it. Strong acids have high K a or small pK a values weak acids have very small K a values or large pK a values. When you have a weak acid you have basically any acid with a formula and commonly you usually see them with an H in the front.
A conjugate acid contains one more H atom and one more charge than the base that formed it. Some examples of weak acids are organic acids molecules with a COOH group such as acetic acid CH3CH2COOH. If it turns red the solution is acidic.
Acetic acid also known as ethanoic acid is a weak acid with the chemical formula CH 3 COOH. You might see it like in HF as an example or in HC2H302 acetic acid. A conjugate base contains one less H atom and one more - charge than the acid that formed it.
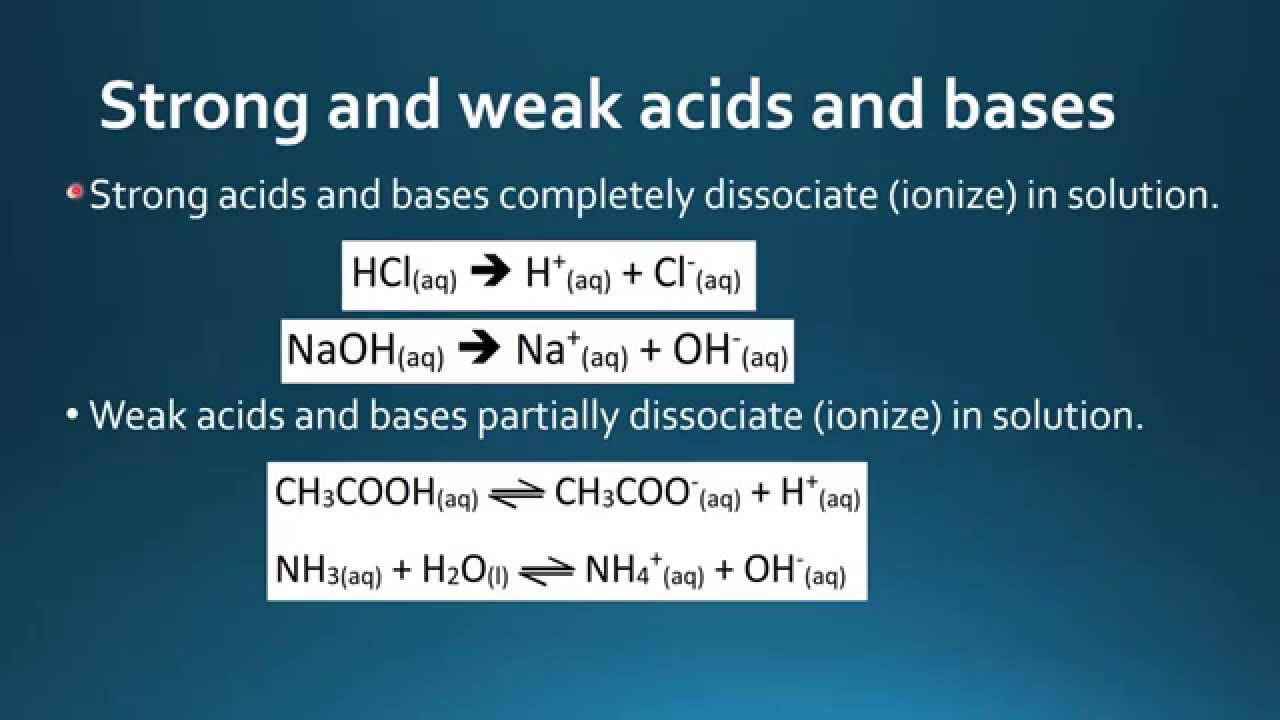
Effect of pH on strength of acids and bases. Strong acidsbases dissociate completely whereas weak acidsbases dissociate partially. A weak acid only partially dissociates in water to give H and the anion.
Acid strength anion size and bond energy. Identifying weak bases and strong bases. This is the currently selected item.
The strength of weak acid is measured by the amount of dislocation in it-the more it dissociates the stronger the acid becomes. Identify key species present in the titration of a weak acid by a strong base or a weak base by a strong acid. The equation representing the ionization of any weak.
But you know if a strong acid is reacting with a weak base then in that case the salt that gets formed takes the nature of the strong parent. It is known to be the active component of vinegar which is a 4 7 solution of acetic acid in water. The lower the Ka or higher the pKa the weaker the acid.
An aqueous solution of a weak acid in a state of equilibrium would consist mainly of the unionized form of the acid and only a small amount of hydronium ions and of the anion conjugate base of the weak acid. This means that a weak acid does not donate all of its hydrogen ions H in a solution. Strong and weak acidsbases.
Such a titration reveals the pKa of the weak acid explains the buffer action of the weak base pair. Weak acids dissociate partially in solution to release H and the concentration of H is much lower than concentration of the weak acidSimiliary weak base. Strength of solution vs.
It is a weak acid if an acid is not mentioned here. And on the other hand when we have a weak acid reacting with a strong base it. The obtained titration plot will then be used to determine the.
A weak acid is one that does not dissociate completely in solution. This is the currently selected item. Let us take the example of bicarbonate ions reacting with water to create carbonic acid and hydronium ions.
Introduction to acidbase reactions. Dip one end of the litmus paper into the solution and then take it out immediately. Acetic acid is a weak acid because it only partially dissociates into its constituent ions when dissolved in water.
Identify the acid and bases as. Here as an example we have selected acetic acid CH 3 COOH as the weak acid and it is titrated against a strong base NaOH. Water autoionization and Kw.
Examples of weak acids include hydrofluoric acid HF and acetic acid CH 3 COOH. So starting with a H in the formula so Hydrogen because thats where the Hydrogen gets donated the H. Weak acids are acids that do not dissociate entirely in solution.
They have a pH value of around 3 to 7. Write a balanced chemical equation for the reaction. These weak acids are all compounds that are uncharged in their protonated state.
Every acid that is 100 percent dissociated from ions is considered a solid acid. Steps on How to Identify the Major Species in a Mixture of Weak and Strong Acids or Bases. A weak acid is any acid that reacts with water donates H ions to a very small extent usually less than 5 - 10.
The weaker acid is the more difficult it is to lose the H ions. Identifying weak acids and strong acids. The purpose of this exercise is to identify an unknown weak acid by titration with a standard sodium hydroxide solution.
You can use the acid equilibrium constant K a or pK a to determine whether an acid is strong or weak. Weak acids have very small values for K a and therefore higher values for pK a compared to strong acids which have very large K a values and slightly negative pK a values. For example formic acid is HCOOH R H acetic acid is CH 3 COOH R CH 3.
Lets look at some more examples of weak acids. Other weak acids are HF H2CO3 H3PO4 and many more. Need help with chemistry.

Acids And Bases I Introduction
8 3 3 Distinguish Between Strong And Weak Acids And Bases Youtube


0 Comments